Way remains murky for MDMA assisted therapy
Katrina Borthwick - 16th September 2024
MDMA assisted psychotherapy is looking a little rocky lately. The Journal of Psychopharmacology has recently retracted three research papers supporting the treatment over data integrity concerns, and the FDA has rejected it as a treatment for PTSD.
Our very own Mark Honeychurch has volunteered to do his own trial, in the interests of science, and has applied for the NZ Skeptics society’s small grants funding to support this. Unfortunately, it appears the sample size (just himself) may be a little too small, and there are some legal and ethical questions that need to be resolved. Add to this, Mark doesn’t really know where to buy it.
Sorry Mark, nice try - stick to the tea.
What is MDMA assisted psychotherapy?
The street name for MDMA is Ecstasy (or Molly) and it is usually taken in pill form or snorted. It is illegal in New Zealand and most other countries.
MDMA assisted psychotherapy proposes to use prescribed doses of MDMA in addition to psychotherapy sessions, in the hope of improving the effectiveness of those sessions. It is touted primarily for Post Traumatic Stress Disorder (PTSD), but there are those who advocate its use for other mental health disorders. These are low doses we are talking about, and there isn’t really any solid research on microdosing of MDMA. Microdosing is where doses are so small they are not supposed to have any perceived effects.
There is some discussion in New Zealand on whether legalising and regulating MDMA would make it safer. This would ensure that what is taken is unadulterated, and that good safety information is provided to consumers – for example on the possible interactions with other legal or illegal substances. MDMA does increase blood pressure and heart rate, and can have some rarer effects on some people that are life threatening.
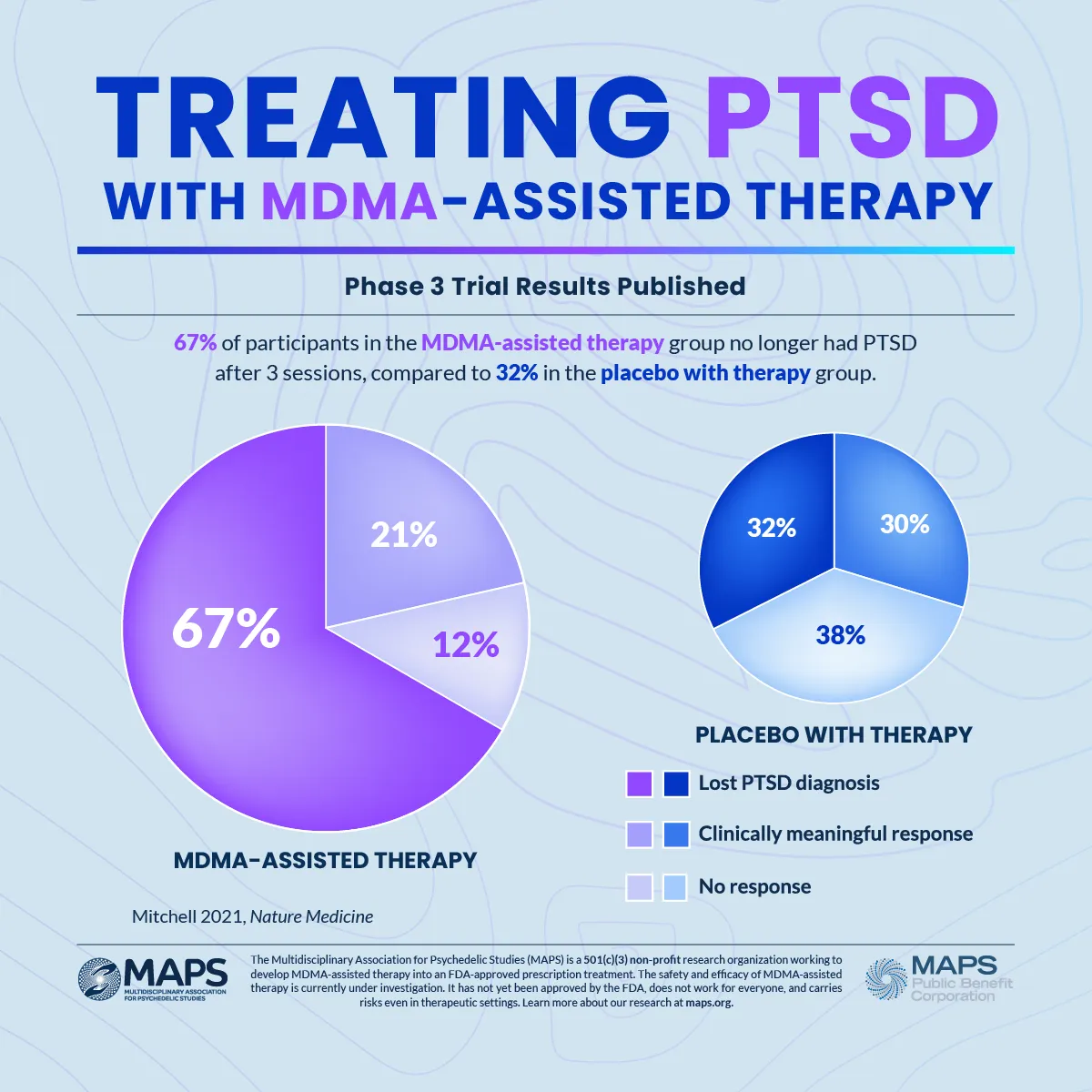
What are the issues?
To date, no psychedelic medicines have been approved by the FDA. So, this would have been the first.
The rejection of the FDA application by Lykos (previously called MAPS) referred to issues with the study design, as well as missing data, and they said that an additional clinical trial would be required. Several of the authors of the retracted papers were affiliated with Lykos (MAPS), and this conflict of interest was not fully declared.
“Several of the authors are affiliated with either the Multidisciplinary Association for Psychedelic Studies (MAPS) or MAPS Public Benefit Corporation (MAPS PBC), a subsidiary that is wholly owned by MAPS. As is stated in the Funding declaration, MAPS fully funded and provided the MDMA that was used in this trial, and MAPS PBC organised the trial.”
The drug trials have also faced some scandal around them, including an alleged sexual assault by a therapist during a treatment in 2015, and a relationship between a researcher and trial participant. There had also been a failure to follow the usual practice when using psychedelics in research of having two researchers present. Lykos publicly acknowledged this in 2019 and reported it to the FDA, but failed to notify the journal of the ethics violation prior to publication, or to remove the data generated from the studies.
Added to this is a concern by FDA Advisors that the second phase 3 trial was not really blind. Over 90% of those in the MDMA group, and 75% in the placebo group, correctly guessed if they were taking MDMA or not. Albeit this is a common problem with psychoactive drugs trials, and the FDA had approved the study design.
Another issue was that it appears that 40% of participants had taken MDMA before, and may have actively sought out the trial. These participants were likely to have had a positive view of MDMA.
The possibility of expectation bias being able to explain the small, but statistically significant, difference between the treatment groups is a real problem. The FDA needs to be sure there is a benefit when approving a drug, and that the benefit outweighs any potential harms. In this case the data wasn’t clear enough in that regard, due to these issues.

Where to now?
The general sense is that there may be a possible pathway to proving the beneficial use of MDMA assisted therapy, but we just don’t have the research we need to support this yet. Until that occurs, this treatment isn’t going to get past the safety checks And this is going to be really challenging, due to the issues of blinding. It is pretty hard to come up with a placebo that people believe is MDMA, particularly in a situation where the participants have previously experienced the drug.