Trans-Tasman Troublemaking
Mark Honeychurch - 29th April 2024
Last week I went to Australia for a holiday. Although tourist traps are not my idea of a good holiday destination, I have a family, and when you’re a parent, holidays tend to be more about the kids than your own preferences. And so off we went to the Gold Coast - a place that was both warm and had lots of theme parks.
On the Saturday evening we visited the Surfers Paradise Night Markets, where the stalls sold a wide range of touristy and arty products - wooden sunglasses, a photo opportunity with a snake, candles, jewellery, etc. Just as we were finishing up, having walked the length of the market, two of the very last stalls piqued my interest:





The copper bracelet was being sold as a small part of a larger stall, and I didn’t get the details of the stall name - so I figured there’s not much I could do about that one. But the Pain Free stall (run by a company called NoPainClub), selling an “Active Chilli Concentrate” herbal therapy that can treat the pain from “Athritis (sic), Sciatica, Nerve Damage, Slipped Discs and Sore Knees” - that I was pretty sure I would be able to complain about.
So, once I arrived back in New Zealand, I looked up Australia’s equivalent of our Advertising Standards Authority. For Australia, the organisation has a shortened name of “Ad Standards” - oh how they love to shorten their words over there! I checked the Ad Standards site and, unlike our local ASA, there didn’t seem to be any kind of therapeutic code; just a code of Ethics, which didn’t quite cover the unproven claims I had seen, and codes for food, ads for kids, environmental claims (I wish we had one of those over here), gambling and cars:

This was a little worrying, until I found a page on the Ad Standards site titled “What We Cover”. On that page, it said:
Issues not covered by the advertising industry codes
…
Therapeutic goods
The Therapeutic Goods Administration (TGA) regulates ads for therapeutic goods. All advertising of therapeutic goods is subject to the requirements of the Therapeutic Goods Act 1989 (the Act) and the Therapeutic Goods Regulations 1990 (the Regulations). Visit the TGA website to find out more.
You can make a complaint to Ad Standards about the content of a therapeutic goods ad when there is an issue that falls within the advertising codes (e.g. the ad features violence, offensive language, nudity etc).
Okay, so it looked like I had to go to the TGA website to make my complaint. After clicking on the link, there was a button labelled “Report a non-compliant advertisement” that took me to a handy five page form that I could fill out:
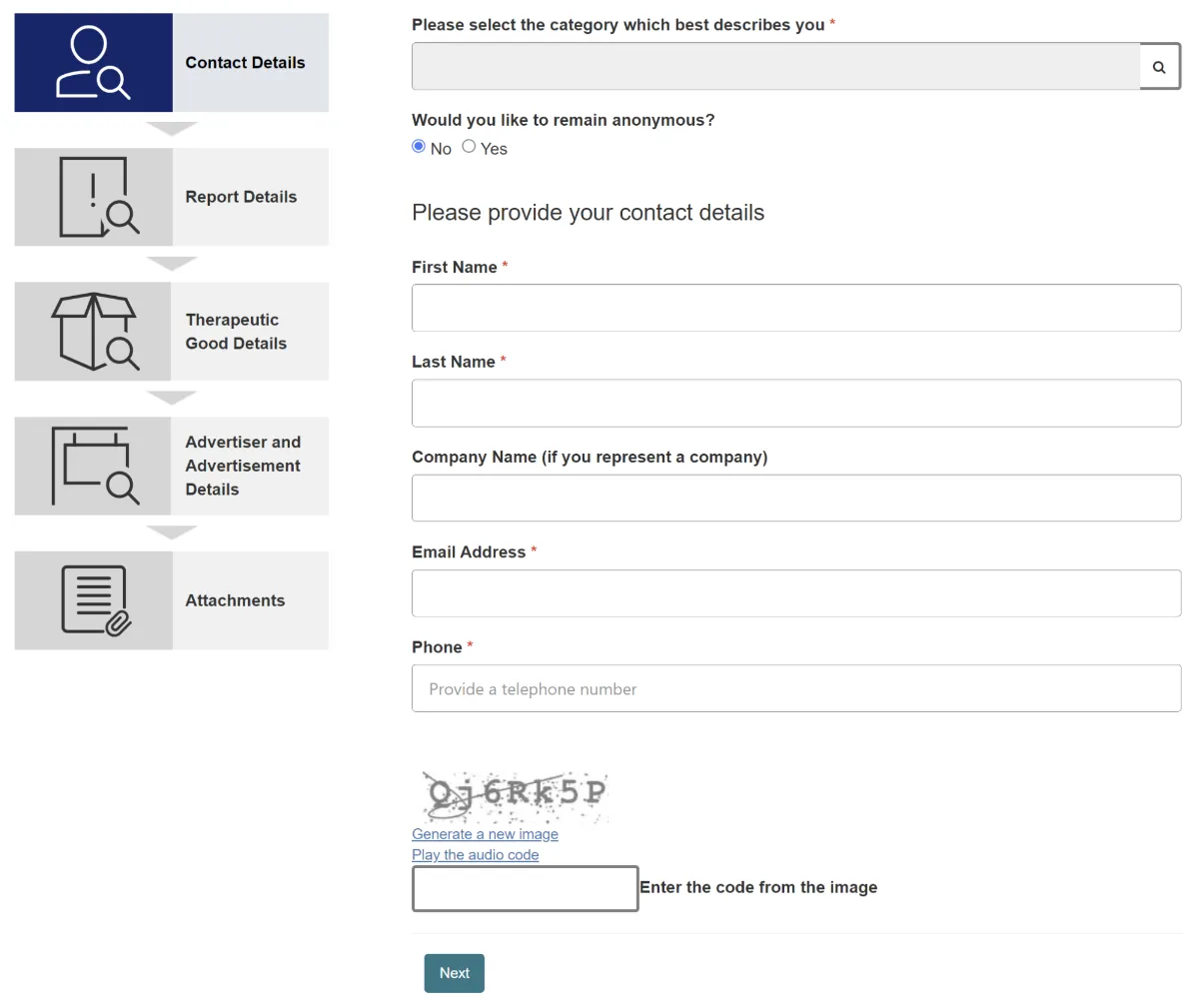
The form had some useful info at the top, such as what an advert is (which isn’t as obvious as it seems), what types of products are covered by the complaint form, and even a freephone 1800 number to get help with filling out the form if I needed it.
The first page of the form simply asked for my contact details, but the second page had an interesting set of concerns about the advert that I had to pick one of:
- The therapeutic good is being advertised with therapeutic claims but is not in the ARTG
- The therapeutic good is advertised as “TGA approved”
- The therapeutic good being advertised is restricted or prohibited from being advertised
- The advertising of the therapeutic good is misleading
- Other concerns
The ARTG in the first option is the “Australian Register of Therapeutic Goods” - a searchable list (as of the 28th of April 2024) of 96,677 registered products, along with information including the claims that are allowed to be made about them. For example, the very first product on the list, “ApoHealth Saline Nasal Spray”, is allowed to make claims about being able to:
- Decrease/reduce/relieve post nasal drip
- Unblock/clear nasal passages
- Helps enhance/improve nose breathing
- Relieves dry nose
- Helps decrease/reduce/relieve nasal itching
- Relieve runny/dripping nose
Sadly not all products stick to proven claims, and herbal products with ingredients like “Curcuma longa” (turmeric), “hemp seed oil” and “Piper nigrum” (black pepper) are routinely being allowed to make claims like:
- Traditionally used in Western herbal medicine to anti-inflammatory/relieve inflammation
- Traditionally used in Western herbal medicine to analgesic/Anodyne/relieve pain
- Traditionally used in Western herbal medicine to decrease/reduce/relieve symptoms of soft tissue trauma
To me this seems like a bad idea, allowing the use of phrases about products being “Traditionally used in” “Ayurvedic medicine”, “Western herbal medicine”, “Chinese medicine”, “Aromatherapy” and more. Evidence of traditional use is not evidence of efficacy; end of story.
I was also concerned about the inclusion of fluffy claims like “Helps reduce/decrease free radical damage to body cells”, and weasel words that are sadly allowed in New Zealand advertising as well as in Australia, such as in the allowable claim “Maintain/support joint health”. Honestly, that sentence really says nothing about the product’s ability to do anything actually beneficial for joints, but there’s a real risk that the average consumer would read it and think that, because it mentions “joint health”, the product will help with their joint issues.
The way these weasel words such as “supports” and “maintains” work is that it’s argued that technically anything providing nutrient value to the human body is, however indirectly, supporting or maintaining joint health. But, if that’s the case, then a cheeseburger also supports joint health, and I think people would be generally unhappy with the idea of Hungry Jack’s (Burger King for non-Australians!) slapping those kinds of claims on their billboards. So, if we agree that we shouldn’t go around telling people that a cheeseburger can help them with their joint health, we really shouldn’t be allowing anyone to do the same for unproven herbal remedies either.
A quick search for “NoPainClub” on the ARTG website came back with 0 results, although a more permissive search for “No Pain” (either with or without quotes) returned 325 results, none of which was what I was looking for. Sadly their search functionality is a little basic, and with nearly 100,000 products in the register it’s likely to be an uphill slog for anyone to find what they’re looking for as any search that’s not hyper-specific or doesn’t contain unique words is likely to return a bunch of irrelevant results.
Having confirmed with my search that the No Pain product I want to complain about is not on the Australian Register of Therapeutic Goods, I proceeded to tick the “concern” from the given list that “The advertising of the therapeutic good is misleading”. I was then asked to describe my concerns in a textarea box, into which I decided to keep it short and sweet and wrote:
At the Surfers Paradise Night Market, stall advertising for the NoPainClub’s “No Pain” Herbal Therapy product with “Active Chilli Concentrate” makes claims about the product’s ability to relieve pain associated with a variety of conditions, including Arthritis, Sciatica, Slipped Discs, Fibromyalgia, Nerve Damage, Tendinitis, Bursitis, Fractures, Osteoporosis and more. As far as I can see, none of these claims has been backed up by any kind of scientific evidence, and so it seems wrong to be making these kinds of claims to unsuspecting consumers.
The next page of the form asked me what category of product I was reporting, giving me the options of:
- Biological Products
- Complementary and Homeopathic Preparations
- Medical Devices
- OTC Medicines
- Other Therapeutic Good
- Prescription Medicines
- Unregistered Goods
I figured that this product, with its herbal ingredients, falls under the first, second and last of these. I ended up choosing the second, “Complementary and Homeopathic Preparations”, as it seemed the most fitting.
The next field, which was good to see, was the ability to find a product in the ARTG and add it to a list of products I was reporting. As the product was not registered when I searched for it, I skipped this and instead just had to copy and paste the product name from my complaint text to a text box.
In the fourth page of the form I had to supply the company name (NoPainClub), the type of advertisement (there was a four page list of options, such as TV, Radio, internet, etc, from which I chose “Poster”), and where and when I saw the advertisement (Surfers Paradise Night Market, 20th April 2024). I also had to say whether I’d complained about the advertisement anywhere else.
The final page of the form allowed me to upload attachments, and I supplied the three images I had of the No Pain posters. I clicked the Submit button, and was greeted with a confirmation that my complaint had been received:
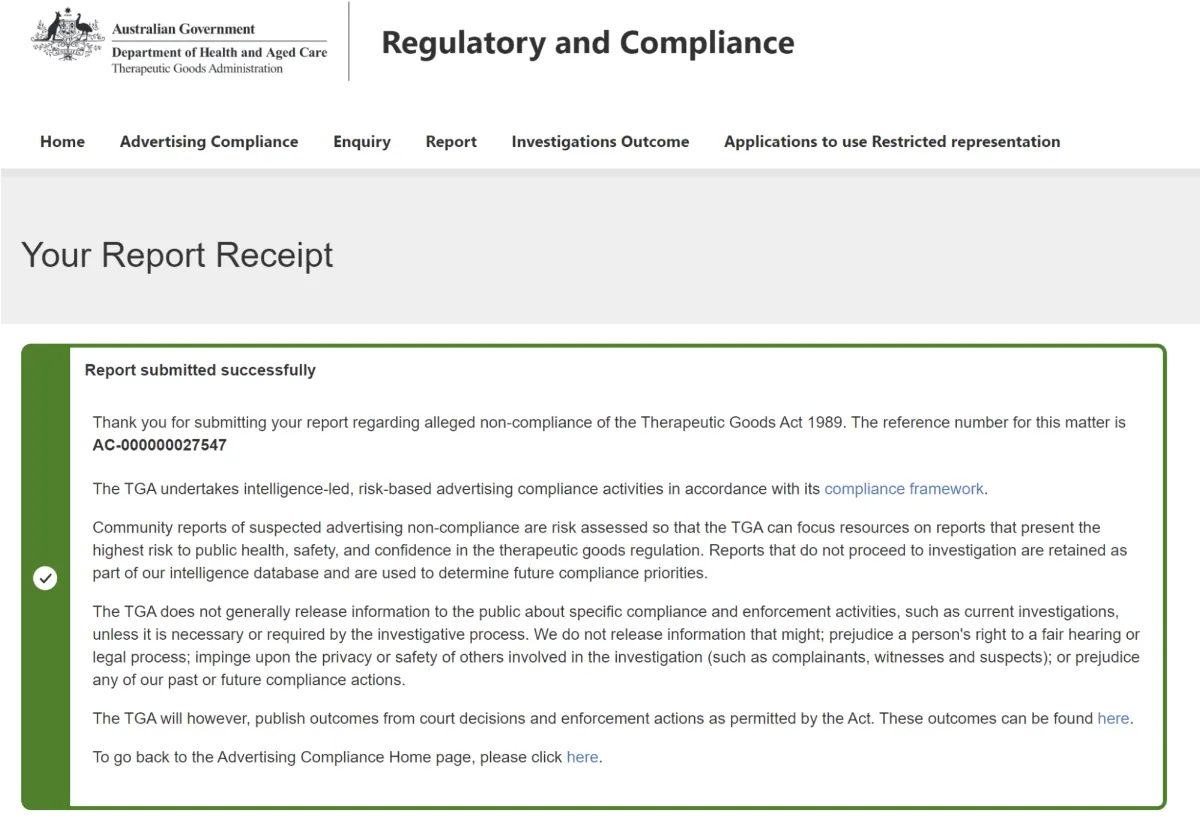
Having made a LOT of New Zealand Advertising Standards complaints in the past 10 years, I have to say that I think the Australian Therapeutic Goods complaint form is less janky and onerous, although I’ll have to see what happens with my complaint before I know whether this process is ultimately worth utilising. One of the good things about New Zealand’s ASA is that they will consider all complaints they receive, and make a judgement on a complaint if there’s any merit to it, but I’m not sure if Australia’s Department of Health and Aged Care is legally required to do anything with my complaint if they don’t want to. It’s quite possible they’ll see it as not worth their time and effort, and just quietly ignore it. But, at least I’ve done my bit, and as a bonus I managed to make my kids despair that, even on holiday, I was still being boringly skeptical and watching out for bogus health claims. And, if the complaint is successful, I may well make a second complaint about the company’s Magnesium Oil in the near future.